Part 5: Neonatal Resuscitation
- Newborn resuscitation requires anticipation and preparation by providers who train individually and as teams.
- Most newly born infants do not require immediate cord clamping or resuscitation and can be evaluated and monitored during skin-to-skin contact with their mothers after birth.
- Inflation and ventilation of the lungs are the priority in newly born infants who need support after birth.
- A rise in heart rate is the most important indicator of effective ventilation and response to resuscitative interventions.
- Pulse oximetry is used to guide oxygen therapy and meet oxygen saturation goals.
- Chest compressions are provided if there is a poor heart rate response to ventilation after appropriate ventilation corrective steps, which preferably include endotracheal intubation.
- The heart rate response to chest compressions and medications should be monitored electrocardiographically.
- If the response to chest compressions is poor, it may be reasonable to provide epinephrine, preferably via the intravenous route.
- Failure to respond to epinephrine in a newborn with history or examination consistent with blood loss may require volume expansion.
- If all these steps of resuscitation are effectively completed and there is no heart rate response by 20 minutes, redirection of care should be discussed with the team and family
It is estimated that approximately 10% of newly born infants need help to begin breathing at birth,1–3 and approximately 1% need intensive resuscitative measures to restore cardiorespiratory function.4,5 The neonatal mortality rate in the United States and Canada has fallen from almost 20 per 1000 live births 6,7 in the 1960s to the current rate of approximately 4 per 1000 live births. The inability of newly born infants to establish and sustain adequate or spontaneous respiration contributes significantly to these early deaths and to the burden of adverse neurodevelopmental outcome among survivors. Effective and timely resuscitation at birth could therefore improve neonatal outcomes further.
Successful neonatal resuscitation efforts depend on critical actions that must occur in rapid succession to maximize the chances of survival. The International Liaison Committee on Resuscitation (ILCOR) Formula for Survival emphasizes 3 essential components for good resuscitation outcomes: guidelines based on sound resuscitation science, effective education of resuscitation providers, and implementation of effective and timely resuscitation.8 The 2020 neonatal guidelines contain recommendations, based on the best available resuscitation science, for the most impactful steps to perform in the birthing room and in the neonatal period. In addition, specific recommendations about the training of resuscitation providers and systems of care are provided in their respective guideline Parts.9,10
This guideline is designed for North American healthcare providers who are looking for an up-to-date summary for clinical care, as well as for those who are seeking more in-depth information on resuscitation science and gaps in current knowledge. The science of neonatal resuscitation applies to newly born infants transitioning from the fluid-filled environment of the womb to the air-filled environment of the birthing room and to newborns in the days after birth. In circumstances of altered or impaired transition, effective neonatal resuscitation reduces the risk of mortality and morbidity. Even healthy babies who breathe well after birth benefit from facilitation of normal transition, including appropriate cord management and thermal protection with skin-to-skin care.
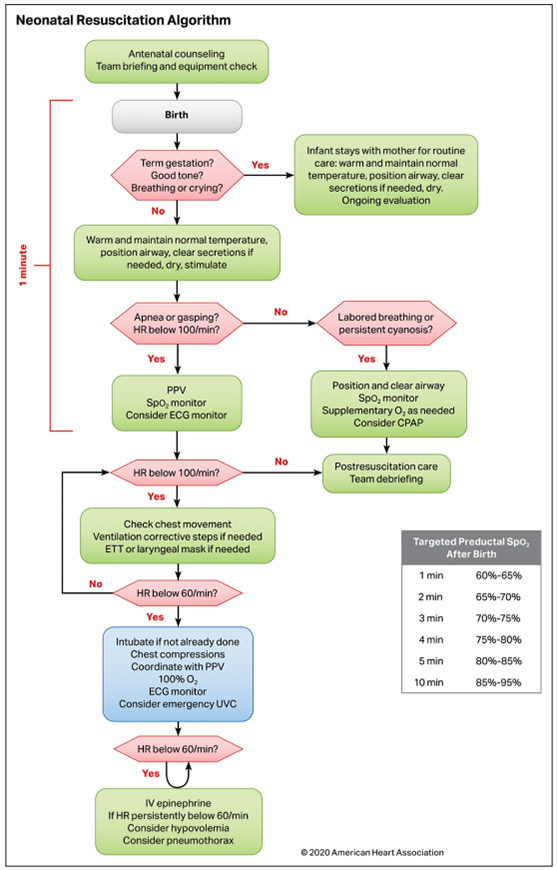
The 2015 Neonatal Resuscitation Algorithm and the major concepts based on sections of the algorithm continue to be relevant in 2020 (Figure(link opens in new window)(link opens in new window)). The following sections are worth special attention.
- Positive-pressure ventilation (PPV) remains the main intervention in neonatal resuscitation. While the science and practices surrounding monitoring and other aspects of neonatal resuscitation continue to evolve, the development of skills and practice surrounding PPV should be emphasized.
- Supplemental oxygen should be used judiciously, guided by pulse oximetry.
- Prevention of hypothermia continues to be an important focus for neonatal resuscitation. The importance of skin-to-skin care in healthy babies is reinforced as a means of promoting parental bonding, breast feeding, and normothermia.
- Team training remains an important aspect of neonatal resuscitation, including anticipation, preparation, briefing, and debriefing. Rapid and effective response and performance are critical to good newborn outcomes.
- Delayed umbilical cord clamping was recommended for both term and preterm neonates in 2015. This guideline affirms the previous recommendations.
- The 2015 American Heart Association (AHA) Guidelines Update for Cardiopulmonary Resuscitation (CPR) and Emergency Cardiovascular Care (ECC) recommended against routine endotracheal suctioning for both vigorous and nonvigorous infants born with meconium-stained amniotic fluid (MSAF). This guideline reinforces initial steps and PPV as priorities.
It is important to recognize that there are several significant gaps in knowledge relating to neonatal resuscitation. Many current recommendations are based on weak evidence with a lack of well-designed human studies. This is partly due to the challenges of performing large randomized controlled trials (RCTs) in the delivery room. The current guideline, therefore, concludes with a summary of current gaps in neonatal research and some potential strategies to address these gaps.
COVID-19 Guidance
Together with other professional societies, the AHA has provided interim guidance for basic and advanced life support in adults, children, and neonates with suspected or confirmed coronavirus disease 2019 (COVID-19) infection. Because evidence and guidance are evolving with the COVID-19 situation, this interim guidance is maintained separately from the ECC guidelines. Readers are directed to the AHA website for the most recent guidance.12
Open table in a new window
Each AHA writing group reviewed all relevant and current AHA guidelines for CPR and ECC18–20 and all relevant 2020 ILCOR International Consensus on CPR and ECC Science With Treatment Recommendations evidence and recommendations21 to determine if current guidelines should be reaffirmed, revised, or retired, or if new recommendations were needed. The writing groups then drafted, reviewed, and approved recommendations, assigning to each a Level of Evidence (LOE; ie, quality) and Class of Recommendation (COR; ie, strength) (Table(link opens in new window)).11
These guidelines apply primarily to the “newly born” baby who is transitioning from the fluid-filled womb to the air-filled room. The “newly born” period extends from birth to the end of resuscitation and stabilization in the delivery area. However, the concepts in these guidelines may be applied to newborns during the neonatal period (birth to 28 days).
The primary goal of neonatal care at birth is to facilitate transition. The most important priority for newborn survival is the establishment of adequate lung inflation and ventilation after birth. Consequently, all newly born babies should be attended to by at least 1 person skilled and equipped to provide PPV. Other important goals include establishment and maintenance of cardiovascular and temperature stability as well as the promotion of mother-infant bonding and breast feeding, recognizing that healthy babies transition naturally.
The Neonatal Resuscitation Algorithm remains unchanged from 2015 and is the organizing framework for major concepts that reflect the needs of the baby, the family, and the surrounding team of perinatal caregivers.
Anticipation and Preparation
Every healthy newly born baby should have a trained and equipped person assigned to facilitate transition. Identification of risk factors for resuscitation may indicate the need for additional personnel and equipment. Effective team behaviors, such as anticipation, communication, briefing, equipment checks, and assignment of roles, result in improved team performance and neonatal outcome.
Cord Management
After an uncomplicated term or late preterm birth, it is reasonable to delay cord clamping until after the baby is placed on the mother, dried, and assessed for breathing, tone, and activity. In other situations, clamping and cutting of the cord may also be deferred while respiratory, cardiovascular, and thermal transition is evaluated and initial steps are undertaken. In preterm birth, there are also potential advantages from delaying cord clamping.
Initial Actions
When possible, healthy term babies should be managed skin-to-skin with their mothers. After birth, the baby should be dried and placed directly skin-to-skin with attention to warm coverings and maintenance of normal temperature. There should be ongoing evaluation of the baby for normal respiratory transition. Radiant warmers and other warming adjuncts are suggested for babies who require resuscitation at birth, especially very preterm and very low-birth-weight babies. Stimulation may be provided to facilitate respiratory effort. Suctioning may be considered for suspected airway obstruction.
Assessment of Heart Rate
Heart rate is assessed initially by auscultation and/or palpation. Oximetry and electrocardiography are important adjuncts in babies requiring resuscitation.
Positive-Pressure Ventilation
PPV remains the primary method for providing support for newborns who are apneic, bradycardic, or demonstrate inadequate respiratory effort. Most babies will respond to this intervention. An improvement in heart rate and establishment of breathing or crying are all signs of effective PPV.
Oxygen Therapy
PPV may be initiated with air (21% oxygen) in term and late preterm babies, and up to 30% oxygen in preterm babies. Oximetry is used to target the natural range of oxygen saturation levels that occur in term babies.
Chest Compressions
If the heart rate remains less than 60/min despite 30 seconds of adequate PPV, chest compressions should be provided. The suggested ratio is 3 chest compressions synchronized to 1 inflation (with 30 inflations per minute and 90 compressions per minute) using the 2 thumb–encircling hands technique for chest compressions.
Vascular Access
When vascular access is required in the newly born, the umbilical venous route is preferred. When intravenous access is not feasible, the intraosseous route may be considered.
Medications
If the heart rate remains less than 60/min despite 60 seconds of chest compressions and adequate PPV, epinephrine should be administered, ideally via the intravenous route.
Volume Expansion
When blood loss is known or suspected based on history and examination, and there is no response to epinephrine, volume expansion is indicated.
Withholding and Discontinuing Resuscitation
It may be possible to identify conditions in which withholding or discontinuation of resuscitative efforts may be reasonably considered by families and care providers. Appropriate and timely support should be provided to all involved.
Human Factors and Systems
Teams and individuals who provide neonatal resuscitation are faced with many challenges with respect to the knowledge, skills, and behaviors needed to perform effectively. Neonatal resuscitation teams may therefore benefit from ongoing booster training, briefing, and debriefing.
| Abbreviation |
Meaning/Phrase |
|---|---|
| AHA | American Heart Association |
| COR | Class of Recommendation |
| CPAP | continuous positive airway pressure |
| ECC | emergency cardiovascular care |
| ECG | electrocardiogram/electrocardiographic |
| H20 | water |
| HIE | hypoxic-ischemic encephalopathy |
| ILCOR | International Liaison Committee on Resuscitation |
| LOE | Level of Evidence |
| MSAF | meconium-stained amniotic fluid |
| PEEP | positive end-expiratory pressure |
| PPV | positive pressure ventilation |
| RCT | randomized controlled trial |
| ROSC | return of spontaneous circulation |
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR |
|
| 1 | B-NR |
|
| 1 | C-LD | |
| 1 | C-LD |
Synopsis
Approximately 10% of newborns require assistance to breathe after birth.1–3,5,13 Newborn resuscitation requires training, preparation, and teamwork. When the need for resuscitation is not anticipated, delays in assisting a newborn who is not breathing may increase the risk of death.1,5,13 Therefore, every birth should be attended by at least 1 person whose primary responsibility is the newborn and who is trained to begin PPV without delay.2–4
A risk assessment tool that evaluates risk factors present during pregnancy and labor can identify newborns likely to require advanced resuscitation; in these cases, a team with more advanced skills should be mobilized and present at delivery.5,7 In the absence of risk stratification, up to half of babies requiring PPV may not be identified before delivery.6,13
A standardized equipment checklist is a comprehensive list of critical supplies and equipment needed in a given clinical setting. In the birth setting, a standardized checklist should be used before every birth to ensure that supplies and equipment for a complete resuscitation are present and functional.8,9,14,15
A predelivery team briefing should be completed to identify the leader, assign roles and responsibilities, and plan potential interventions. Team briefings promote effective teamwork and communication, and support patient safety.8,10–12
Recommendation-Specific Supportive Text
- A large observational study found that delaying PPV increases risk of death and prolonged hospitalization.1 A systematic review and meta analysis showed neonatal resuscitation training reduced stillbirths and improved 7-day neonatal survival in low-resource countries.3 A retrospective cohort study demonstrated improved Apgar scores among high-risk newborns after neonatal resuscitation training.16
- A multicenter, case-control study identified 10 perinatal risk factors that predict the need for advanced neonatal resuscitation.7 An audit study done before the use of risk stratification showed that resuscitation was anticipated in less than half of births requiring PPV.6 A prospective cohort study showed that risk stratification based on perinatal risk factors increased the likelihood of skilled team attendance at high-risk births.5
- A multicenter quality improvement study demonstrated high staff compliance with the use of a neonatal resuscitation bundle that included briefing and an equipment checklist.8 A management bundle for preterm infants that included team briefing and equipment checks resulted in clear role assignments, consistent equipment checks, and improved thermoregulation and oxygen saturation.9
- A single-center RCT found that role confusion during simulated neonatal resuscitation was avoided and teamwork skills improved by conducting a team briefing.11 A statewide collaborative quality initiative demonstrated that team briefing improved team communication and clinical outcomes.10 A single-center study demonstrated that team briefing and an equipment checklist improved team communication but showed no improvement in equipment preparation.12
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-R |
|
| 2b | C-LD |
|
| 2b | C-EO |
|
| 3: No Benefit | B-R |
|
Synopsis
During an uncomplicated term or late preterm birth, it may be reasonable to defer cord clamping until after the infant is placed on the mother and assessed for breathing and activity. Early cord clamping (within 30 seconds) may interfere with healthy transition because it leaves fetal blood in the placenta rather than filling the newborn’s circulating volume. Delayed cord clamping is associated with higher hematocrit after birth and better iron levels in infancy.9–21 While developmental outcomes have not been adequately assessed, iron deficiency is associated with impaired motor and cognitive development.24–26 It is reasonable to delay cord clamping (longer than 30 seconds) in preterm babies because it reduces need for blood pressure support and transfusion and may improve survival.1–8
There are insufficient studies in babies requiring PPV before cord clamping to make a recommendation.22 Early cord clamping should be considered for cases when placental transfusion is unlikely to occur, such as maternal hemorrhage or hemodynamic instability, placental abruption, or placenta previa.27 There is no evidence of maternal harm from delayed cord clamping compared with early cord clamping.10–12,28–34 Cord milking is being studied as an alternative to delayed cord clamping but should be avoided in babies less than 28 weeks’ gestational age, because it is associated with brain injury.23
Recommendation-Specific Supportive Text
- Compared with preterm infants receiving early cord clamping, those receiving delayed cord clamping were less likely to receive medications for hypotension in a meta-analysis of 6 RCTs1–6 and receive transfusions in a meta-analysis of 5 RCTs.7 Among preterm infants not requiring resuscitation, delayed cord clamping may be associated with higher survival than early cord clamping is.8 Ten RCTs found no difference in postpartum hemorrhage rates with delayed cord clamping versus early cord clamping.10–12,28–34
- Compared with term infants receiving early cord clamping, term infants receiving delayed cord clamping had increased hemoglobin concentration within the first 24 hours and increased ferritin concentration in the first 3 to 6 months in meta-analyses of 12 and 6 RCTs,9–21 respectively. Compared with term and late preterm infants receiving early cord clamping, those receiving delayed cord clamping showed no significant difference in mortality, admission to the neonatal intensive care unit, or hyperbilirubinemia leading to phototherapy in metaanalyses of 4,10,13,29,35 10,10,12,17,19,21,28,31,34,36,37 and 15 RCTs, respectively.9,12,14,18–21,28–30,32–34,38,39 Compared with term infants receiving early cord clamping, those receiving delayed cord clamping had increased polycythemia in metaanalyses of 1310,11,13,14,17,18,21,29,30,33,39–41 and 8 RCTs,9,10,13,19,20,28,30,34 respectively
- For infants requiring PPV at birth, there is currently insufficient evidence to recommend delayed cord clamping versus early cord clamping.
- A large multicenter RCT found higher rates of intraventricular hemorrhage with cord milking in preterm babies born at less than 28 weeks’ gestational age.23
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | |
| 1 | C-EO |
|
| 1 | B-NR |
|
| 2a | B-NR |
Synopsis
Temperature should be measured and recorded after birth and monitored as a measure of quality.1 The temperature of newly born babies should be maintained between 36.5°C and 37.5°C.2 Hypothermia (less than 36°C) should be prevented as it is associated with increased neonatal mortality and morbidity, especially in very preterm (less than 33 weeks) and very low-birthweight babies (less than 1500 g), who are at increased risk for hypothermia.3–5,7 It is also reasonable to prevent hyperthermia as it may be associated with harm.4,6
Recommendation-Specific Supportive Text
- Hypothermia after birth is common worldwide, with a higher incidence in babies of lower gestational age and birth weight.3–5
- There are long-standing worldwide recommendations for routine temperature management for the newborn.2
- In observational studies in both preterm (less than 37 weeks) and low-birth-weight babies (less than 2500 g), the presence and degree of hypothermia after birth is strongly associated with increased neonatal mortality and morbidity.3–5
- Two observational studies found an association between hyperthermia and increased morbidity and mortality in very preterm (moderate quality) and very low-birth-weight neonates (very low quality).4,6
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-R |
|
| 2a | C-LD |
|
| 2a | B-NR | |
| 2b | B-R |
|
| 2b | B-R |
|
| 2b | C-LD |
|
Synopsis
Healthy babies should be skin-to-skin after birth.8 For preterm and low-birth-weight babies or babies requiring resuscitation, warming adjuncts (increased ambient temperature [greater than 23°C], skin-to-skin care, radiant warmers, plastic wraps or bags, hats, blankets, exothermic mattresses, and warmed humidified inspired gases)10,11,14 individually or in combination may reduce the risk of hypothermia. Exothermic mattresses have been reported to cause local heat injury and hyperthermia.15
When babies are born in out-of-hospital, resource-limited, or remote settings, it may be reasonable to prevent hypothermia by using a clean food-grade plastic bag13 as an alternative to skin-to-skin contact.8
Recommendation-Specific Supportive Text
- A systematic review (low to moderate certainty) of 6 RCTs showed that early skin-to-skin contact promotes normothermia in healthy neonates.8 Two meta-analyses reviewed RCTs and observational studies of extended skin-to-skin care after initial resuscitation and/or stabilization, some in resource-limited settings, showing reduced mortality, improved breastfeeding, shortened length of stay, and improved weight gain in preterm and low-birth-weight babies (moderate quality evidence).16,17
- Most RCTs in well-resourced settings would routinely manage at-risk babies under a radiant warmer.11
- RCTs and observational studies of warming adjuncts, alone and in combination, demonstrate reduced rates of hypothermia in very preterm and very low-birth-weight babies.10,11 However, meta-analysis of RCTs of interventions that reduce hypothermia in very preterm or very low-birthweight babies (low certainty) show no impact on neonatal morbidity or mortality.11 Two RCTs and expert opinion support ambient temperatures of 23°C and above.2,14,18
- One moderate quality RCT found higher rates of hyperthermia with exothermic mattresses.15
- Numerous nonrandomized quality improvement (very low to low certainty) studies support the use of warming adjunct “bundles.”12
- One RCT in resource-limited settings found that plastic coverings reduced the incidence of hypothermia, but they were not directly compared with uninterrupted skin-to-skin care.13
Synopsis
The immediate care of newly born babies involves an initial assessment of gestation, breathing, and tone. Babies who are breathing well and/or crying are cared for skin-to-skin with their mothers and should not need interventions such as routine tactile stimulation or suctioning, even if the amniotic fluid is meconium stained.7,19 Avoiding unnecessary suctioning helps prevent the risk of induced bradycardia as a result of suctioning of the airway.
Recommendation-Specific Supportive Text
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-NR | |
| 2b | C-EO |
|
Synopsis
If there is ineffective breathing effort or apnea after birth, tactile stimulation may stimulate breathing. Tactile stimulation should be limited to drying an infant and rubbing the back and soles of the feet.21,22 There may be some benefit from repeated tactile stimulation in preterm babies during or after providing PPV, but this requires further study.23 If, at initial assessment, there is visible fluid obstructing the airway or a concern about obstructed breathing, the mouth and nose may be suctioned. Suction should also be considered if there is evidence of airway obstruction during PPV
Recommendation-Specific Supportive Text
- Limited observational studies suggest that tactile stimulation may improve respiratory effort. One RCT (low certainty of evidence) suggests improved oxygenation after resuscitation in preterm babies who received repeated tactile stimulation.23
- Suctioning for suspected airway obstruction during PPV is based on expert opinion.7
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-EO |
|
| 3: No Benefit | C-LD |
|
Synopsis
Direct laryngoscopy and endotracheal suctioning are not routinely required for babies born through MSAF but can be beneficial in babies who have evidence of airway obstruction while receiving PPV.7
Recommendation-Specific Supportive Text
- Endotracheal suctioning for apparent airway obstruction with MSAF is based on expert opinion.
- A meta-analysis of 3 RCTs (low certainty of evidence) and a further single RCT suggest that nonvigorous newborns delivered through MSAF have the same outcomes (survival, need for respiratory support, or neurodevelopment) whether they are suctioned before or after the initiation of PPV.7
| COR | LOE | Recommendation |
|---|---|---|
| 2b | C-LD |
|
Synopsis
Auscultation of the precordium remains the preferred physical examination method for the initial assessment of the heart rate.9 Pulse oximetry and ECG remain important adjuncts to provide continuous heart rate assessment in babies needing resuscitation.
ECG provides the most rapid and accurate measurement of the newborn’s heart rate at birth and during resuscitation. Clinical assessment of heart rate by auscultation or palpation may be unreliable and inaccurate.1–4 Compared to ECG, pulse oximetry is both slower in detecting the heart rate and tends to be inaccurate during the first few minutes after birth.5,6,10–12 Underestimation of heart rate can lead to potentially unnecessary interventions. On the other hand, overestimation of heart rate when a newborn is bradycardic may delay necessary interventions. There are limited data comparing the different approaches to heart rate assessment during neonatal resuscitation on other neonatal outcomes. Use of ECG for heart rate detection does not replace the need for pulse oximetry to evaluate oxygen saturation or the need for supplemental oxygen.
Recommendation-Specific Supportive Text
- In one RCT and one observational study, there were no reports of technical difficulties with ECG monitoring during neonatal resuscitation, supporting its feasibility as a tool for monitoring heart rate during neonatal resuscitation.6,7
- One observational study compared neonatal outcomes before (historical cohort) and after implementation of ECG monitoring in the delivery room.8 Compared with the newborns in the historical cohort, newborns with the ECG monitoring had lower rates of endotracheal intubation and higher 5-minute Apgar scores. However, newborns with ECG monitoring also had higher odds of receiving chest compressions in the delivery room.
- Very low-quality evidence from 8 nonrandomized studies2,5,6,10,12–15 enrolling 615 newborns and 2 small RCTs7,16 suggests that at birth, ECG is faster and more accurate for newborn heart assessment compared with pulse oximetry.
- Very low-quality evidence from 2 nonrandomized studies and 1 randomized trial show that auscultation is not as accurate as ECG for heart rate assessment during newborn stabilization immediately after birth.2–4
Synopsis
When chest compressions are initiated, an ECG should be used to confirm heart rate. When ECG heart rate is greater than 60/min, a palpable pulse and/or audible heart rate rules out pulseless electric activity.17–21
Recommendation-Specific Supportive Text
- Given the evidence for ECG during initial steps of PPV, expert opinion is that ECG should be used when providing chest compressions.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR |
|
| 2a | C-LD |
|
| 2b | C-LD |
|
| 3: Harm | C-LD |
Synopsis
The adequacy of ventilation is measured by a rise in heart rate and, less reliably, chest expansion. Peak inflation pressures of up to 30 cm H2O in term newborns and 20 to 25 cm H2O in preterm newborns are usually sufficient to inflate the lungs.5–7,9,11–14 In some cases, however, higher inflation pressures are required.5,7–10 Peak inflation pressures or tidal volumes greater than what is required to increase heart rate and achieve chest expansion should be avoided.24,26–28
The lungs of sick or preterm infants tend to collapse because of immaturity and surfactant deficiency.15 PEEP provides low-pressure inflation of the lungs during expiration. PEEP has been shown to maintain lung volume during PPV in animal studies, thus improving lung function and oxygenation.16 PEEP may be beneficial during neonatal resuscitation, but the evidence from human studies is limited. Optimal PEEP has not been determined, because all human studies used a PEEP level of 5 cm H2O.18–22
Recommendation-Specific Supportive Text
- A large observational study showed that most nonvigorous newly born infants respond to stimulation and PPV. The same study demonstrated that the risk of death or prolonged admission increases 16% for every 30-second delay in initiating PPV.1
- Animal studies in newborn mammals show that heart rate decreases during asphyxia. Ventilation of the lungs results in a rapid increase in heart rate.3,4 Several case series found that most term newborns can be resuscitated using peak inflation pressures of 30 cm H2O, delivered without PEEP.5–8 Occasionally, higher peak pressures are required.5,7–10
- Case series in preterm infants have found that most preterm infants can be resuscitated using PPV inflation pressures in the range of 20 to 25 cm H2O,11–14 but higher pressures may be required.10,11
- An observational study including 1962 infants between 23 and 33 weeks’ gestational age reported lower rates of mortality and chronic lung disease when giving PPV with PEEP versus no PEEP.19
- Two randomized trials and 1 quasi-randomized trial (very low quality) including 312 infants compared PPV with a T-piece (with PEEP) versus a self-inflating bag (no PEEP) and reported similar rates of death and chronic lung disease.20–22 One trial (very low quality) compared PPV using a T-piece and PEEP of 5 cm H2O versus 0 cm H2O and reported similar rates of death and chronic lung disease.23
- Studies of newly born animals showed that PEEP facilitates lung aeration and accumulation of functional residual capacity, prevents distal airway collapse, increases lung surface area and compliance, decreases expiratory resistance, conserves surfactant, and reduces hyaline membrane formation, alveolar collapse, and the expression of proinflammatory mediators.16,18
- One observational study in newly born infants associated high tidal volumes during resuscitation with brain injury.25
- Several animal studies found that ventilation with high volumes caused lung injury, impaired gas exchange, and reduced lung compliance in immature animals.24,26–28
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-EO |
|
| 2a | C-LD |
|
| 3: Harm | B-R |
|
Synopsis
It is reasonable to initiate PPV at a rate of 40 to 60/min to newly born infants who have ineffective breathing, are apneic, or are persistently bradycardic (heart rate less than 100/min) despite appropriate initial actions (including tactile stimulation).1
To match the natural breathing pattern of both term and preterm newborns, the inspiratory time while delivering PPV should be 1 second or less. While there has been research to study the potential effectiveness of providing longer, sustained inflations, there may be potential harm in providing sustained inflations greater than 10 seconds for preterm newborns. The potential benefit or harm of sustained inflations between 1 and 10 seconds is uncertain.2,29
Recommendation-Specific Supportive Text
- Providing PPV at a rate of 40 to 60 inflations per minute is based on expert opinion.
- The ILCOR task force review, when comparing PPV with sustained inflation breaths, defined PPV to have an inspiratory time of 1 second or less, based on expert opinion. One observational study describes the initial pattern of breathing in term and preterm newly born infants to have an inspiratory time of around 0.3 seconds.2
- Two systematic reviews29,30 in preterm newborns (low to moderate certainty) found no significant benefit from sustained lung inflation over PPV; one review found a higher risk of death in the first 48 hours. One large RCT31 was stopped early when an increased rate of early mortality was identified in babies less than 28 weeks’ gestational age who received sustained inflations; no significant difference was found in the primary outcome of death or bronchopulmonary dysplasia.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | A |
|
Synopsis
Newly born infants who breathe spontaneously need to establish a functional residual capacity after birth.8 Some newly born infants experience respiratory distress, which manifests as labored breathing or persistent cyanosis. CPAP, a form of respiratory support, helps newly born infants keep their lungs open. CPAP is helpful for preterm infants with breathing difficulty after birth or after resuscitation33 and may reduce the risk of bronchopulmonary dysplasia in very preterm infants when compared with endotracheal ventilation.34–36 CPAP is also a less invasive form of respiratory support than intubation and PPV are.
Recommendation-Specific Supportive Text
- Four RCTs and 1 meta-analysis32,34–37 (high quality) showed reduction in the combined outcome of death and bronchopulmonary dysplasia when starting treatment with CPAP compared with intubation and ventilation in very preterm infants (less than 30 weeks of gestation) with respiratory distress (the number needed to prevent was 25). The meta-analysis reported no differences in the individual outcomes of mortality, bronchopulmonary dysplasia, pneumothorax, interventricular hemorrhage, necrotizing enterocolitis, or retinopathy of prematurity.32
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-R |
|
| 2b | C-LD | |
| 3: Harm | B-R |
|
Synopsis
During an uncomplicated delivery, the newborn transitions from the low oxygen environment of the womb to room air (21% oxygen) and blood oxygen levels rise over several minutes. During resuscitation, supplemental oxygen may be provided to prevent harm from inadequate oxygen supply to tissues (hypoxemia).4 However, overexposure to oxygen (hyperoxia) may be associated with harm.5
Term and late preterm newborns have lower shortterm mortality when respiratory support during resuscitation is started with 21% oxygen (air) versus 100% oxygen.1 No difference was found in neurodevelopmental outcome of survivors.1 During resuscitation, pulse oximetry may be used to monitor oxygen saturation levels found in healthy term infants after vaginal birth at sea level.3
In more preterm newborns, there were no differences in mortality or other important outcomes when respiratory support was started with low (50% or less) versus high (greater than 50%) oxygen concentrations.2 Given the potential for harm from hyperoxia, it may be reasonable to start with 21% to 30% oxygen. Pulse oximetry with oxygen targeting is recommended in this population.3
Recommendation-Specific Supportive Text
- A meta-analysis of 5 randomized and quasirandomized trials enrolling term and late preterm newborns showed no difference in rates of hypoxic-ischemic encephalopathy (HIE). Similarly, meta-analysis of 2 quasi-randomized trials showed no difference in moderate-to-severe neurodevelopmental impairment at 1 to 3 years of age1 for newborns administered 21% versus 100% oxygen.1
- Meta-analysis of 10 randomized trials enrolling preterm newborns, including subanalysis of 7 trials reporting outcomes for newborns 28 weeks’ gestational age or less, showed no difference in short-term mortality when respiratory support was started with low compared with high oxygen.2 In the included studies, low oxygen was generally 21% to 30% and high oxygen was always 60% to 100%. Furthermore, no differences were found in long-term mortality, neurodevelopmental outcome, retinopathy of prematurity, bronchopulmonary dysplasia, necrotizing enterocolitis, or major cerebral hemorrhage.2 In a systematic review of 8 trials that used oxygen saturation targeting as a cointervention, all preterm babies in whom respiratory support was initiated with 21% oxygen (air) required supplemental oxygen to achieve the predetermined oxygen saturation target.2 The recommendation to initiate respiratory support with a lower oxygen concentration reflects a preference to avoid exposing preterm newborns to additional oxygen (beyond what is necessary to achieve the predetermined oxygen saturation target) without evidence demonstrating a benefit for important outcomes.3
- Meta-analysis of 7 randomized and quasi-randomized trials enrolling term and late preterm newborns showed decreased short-term mortality when using 21% oxygen compared with 100% oxygen for delivery room resuscitation.1 No studies looked at starting with intermediate oxygen concentrations (ie, 22% to 99% oxygen).
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-EO | |
| 2b | C-EO |
Synopsis
Most newborns who are apneic or have ineffective breathing at birth will respond to initial steps of newborn resuscitation (positioning to open the airway, clearing secretions, drying, and tactile stimulation) or to effective PPV with a rise in heart rate and improved breathing. If the heart rate remains less than 60/min despite these interventions, chest compressions can supply oxygenated blood to the brain until the heart rate rises. Ventilation should be optimized before starting chest compressions, with endotracheal intubation if possible. Chest compressions should be started if the heart rate remains less than 60/min after at least 30 seconds of adequate PPV.1
Oxygen is essential for organ function; however, excess inspired oxygen during resuscitation may be harmful. Although current guidelines recommend using 100% oxygen while providing chest compressions, no studies have confirmed a benefit of using 100% oxygen compared to any other oxygen concentration, including air (21%). However, it may be reasonable to increase inspired oxygen to 100% if there was no response to PPV with lower concentrations. Once return of spontaneous circulation (ROSC) is achieved, the supplemental oxygen concentration may be decreased to target a physiological level based on pulse oximetry to reduce the risks associated with hyperoxia.1,2
Recommendation-Specific Supportive Text
- The initiation of chest compressions in newborn babies with a heart rate less than 60/min is based on expert opinion because there are no clinical or physiological human studies addressing this question.
- A meta-analysis (very low quality) of 8 animal studies (n=323 animals) that compared air with 100% oxygen during chest compressions showed equivocal results.3 Two animal studies (very low quality) compared the tissue oxidative stress or damage between air (21%) and 100% oxygen and reported no difference in brain or lung inflammatory markers.3 The use of 100% oxygen during chest compressions is therefore expert opinion.
| COR | LOE | Recommendations |
|---|---|---|
| 2b | C-EO |
|
| 2b | C-LD |
Synopsis
Chest compressions are a rare event in full-term newborns (approximately 0.1%) but are provided more frequently to preterm newborns.11 When providing chest compressions to a newborn, it may be reasonable to deliver 3 compressions before or after each inflation: providing 30 inflations and 90 compressions per minute (3:1 ratio for 120 total events per minute).
Alternative compression-to-ventilation ratios to 3:1, as well as asynchronous PPV (administration of inflations to a patient that are not coordinated with chest compressions), are routinely utilized outside the newborn period, but the preferred method in the newly born is 3:1 in synchrony. Newer methods of chest compression, using a sustained inflation that maintains lung inflation while providing chest compressions, are under investigation and cannot be recommended at this time outside research protocols.12,13
When providing chest compressions to a newborn, the 2 thumb–encircling hands technique may have benefit over the 2-finger technique with respect to blood pressure generation and provider fatigue. When providing chest compressions with the 2 thumb–encircling hands technique, the hands encircle the chest while the thumbs depress the sternum.1,2 The 2 thumb–encircling hands technique can be performed from the side of the infant or from above the head of the newborn.1 Performing chest compressions with the 2 thumb–encircling hands technique from above the head facilitates placement of an umbilical venous catheter.
Recommendation-Specific Supportive Text
- In animal studies (very low quality), the use of alterative compression-to-inflation ratios to 3:1 (eg, 2:1, 4:1, 5:1, 9:3, 15:2, and continuous chest compressions with asynchronous PPV) are associated with similar times to ROSC and mortality rates.4–8
- In a small number of newborns (n=2) with indwelling catheters, the 2 thumb–encircling hands technique generated higher systolic and mean blood pressures compared with the 2-finger technique.9
- One small manikin study (very low quality), compared the 2 thumb–encircling hands technique and 2-finger technique during 60 seconds of uninterrupted chest compressions. The 2 thumb–encircling hands technique achieved greater depth, less fatigue, and less variability with each compression compared with the 2-finger technique.10
Synopsis
Babies who have failed to respond to PPV and chest compressions require vascular access to infuse epinephrine and/or volume expanders. In the delivery room setting, the primary method of vascular access is umbilical venous catheterization. Outside the delivery room, or if intravenous access is not feasible, the intraosseous route may be a reasonable alternative, determined by the local availability of equipment, training, and experience.
Recommendation-Specific Supportive Text
- Umbilical venous catheterization has been the accepted standard route in the delivery room for decades.2 There are no human neonatal studies to support one route over others.1
- There are 6 case reports indicating local complications of intraosseous needle placement.3–8
- Practitioners outside of the delivery room setting, and when umbilical venous catheterization is not feasible, may secure vascular access with the intraosseous route
| COR | LOE | Recommendations |
|---|---|---|
| 2b | C-LD |
|
| 2b | C-LD |
|
| 2b | C-LD | |
| 2b | C-LD |
*In this situation, “intravascular” means intravenous or intraosseous. Intra-arterial epinephrine is not recommended.
Synopsis
Medications are rarely needed in resuscitation of the newly born infant because low heart rate usually results from a very low oxygen level in the fetus or inadequate lung inflation after birth. Establishing ventilation is the most important step to correct low heart rate. However, if heart rate remains less than 60/min after ventilating with 100% oxygen (preferably through an endotracheal tube) and chest compressions, administration of epinephrine is indicated.
Administration of epinephrine via a low-lying umbilical venous catheter provides the most rapid and reliable medication delivery. The intravenous dose of epinephrine is 0.01 to 0.03 mg/kg, followed by a normal saline flush.4 If umbilical venous access has not yet been obtained, epinephrine may be given by the endotracheal route in a dose of 0.05 to 0.1 mg/kg. The dosage interval for epinephrine is every 3 to 5 minutes if the heart rate remains less than 60/min, although an intravenous dose may be given as soon as umbilical access is obtained if response to endotracheal epinephrine has been inadequate.
Recommendation-Specific Supportive Text
- The very limited observational evidence in human infants does not demonstrate greater efficacy of endotracheal or intravenous epinephrine; however, most babies received at least 1 intravenous dose before ROSC.1,2 In a perinatal model of cardiac arrest using term lambs undergoing transition with asphyxia-induced cardiopulmonary arrest, central venous epinephrine was associated with shorter time to ROSC and higher rates of ROSC than endotracheal epinephrine was.3 Intravenous epinephrine followed by a normal saline flush improves medication delivery.4
- One very limited observational study (human) showed 0.03 mg/kg to be an inadequate endotracheal dose.1 In the perinatal model of cardiac arrest, peak plasma epinephrine concentrations in animals were higher and were achieved sooner after central or low-lying umbilical venous administration compared with the endotracheal route, despite a lower intravenous dose (0.03 mg/ kg intravenous versus 0.1 mg/kg endotracheal route).3
- In one very limited observational study, most infants who received an endotracheal dose achieved ROSC after a subsequent intravenous dose.2 Although the more rapid response to intravenous epinephrine warrants its immediate administration once umbilical access is obtained, repetitive endotracheal doses or higher intravenous doses may result in potentially harmful plasma levels that lead to associated hypertension and tachycardia.5–8
- In one very limited observational study, many infants received multiple doses of epinephrine before ROSC.2 The perinatal model of cardiac arrest documented peak plasma epinephrine concentrations at 1 minute after intravenous administration, but not until 5 minutes after endotracheal administration.3
| COR | LOE | Recommendations |
|---|---|---|
| 2b | C-EO |
|
| 2b | C-EO |
Synopsis
A newly born infant in shock from blood loss may respond poorly to the initial resuscitative efforts of ventilation, chest compressions, and/or epinephrine. History and physical examination findings suggestive of blood loss include a pale appearance, weak pulses, and persistent bradycardia (heart rate less than 60/min). Blood may be lost from the placenta into the mother’s circulation, from the cord, or from the infant.
When blood loss is suspected in a newly born infant who responds poorly to resuscitation (ventilation, chest compressions, and/or epinephrine), it may be reasonable to administer a volume expander without delay. Normal saline (0.9% sodium chloride) is the crystalloid fluid of choice. Uncrossmatched type O, Rh-negative blood (or crossmatched, if immediately available) is preferred when blood loss is substantial.4,5 An initial volume of 10 mL/kg over 5 to 10 minutes may be reasonable and may be repeated if there is inadequate response. The recommended route is intravenous, with the intraosseous route being an alternative.
Recommendation-Specific Supportive Text
- There is no evidence from randomized trials to support the use of volume resuscitation at delivery. One large retrospective review found that 0.04% of newborns received volume resuscitation in the delivery room, confirming that it is a relatively uncommon event.1 Those newborns who received volume resuscitation in the delivery room had lower blood pressure on admission to the neonatal intensive care unit compared with those who did not, indicating that factors other than blood loss may be important.1
- There is insufficient clinical evidence to determine what type of volume expander (crystalloid or blood) is more beneficial during neonatal resuscitation. Extrapolation from studies in hypotensive newborns shortly after birth6–8 and studies in animals (piglets) support the use of crystalloid over albumin expanders5 and blood over crystalloid solutions.4 One review discussed recommendations for the use of volume expanders.2
| COR | LOE | Recommendations |
|---|---|---|
| 1 | A |
|
| 1 | C-EO |
|
| 1 | C-LD |
|
| 2b | C-LD |
|
Synopsis
Newly born infants who receive prolonged PPV or advanced resuscitation (eg, intubation, chest compressions ± epinephrine) should be closely monitored after stabilization in a neonatal intensive care unit or a monitored triage area because these infants are at risk for further deterioration.
Infants 36 weeks’ or greater estimated gestational age who receive advanced resuscitation should be examined for evidence of HIE to determine if they meet criteria for therapeutic hypothermia. Therapeutic hypothermia is provided under defined protocols similar to those used in published clinical trials and in facilities capable of multidisciplinary care and longitudinal follow-up. The impact of therapeutic hypothermia on infants less than 36 weeks’ gestational age with HIE is unclear and is a subject of ongoing research trials.
Hypoglycemia is common in infants who have received advanced resuscitation and is associated with poorer outcomes.8 These infants should be monitored for hypoglycemia and treated appropriately.
Infants with unintentional hypothermia (temperature less than 36°C) immediately after stabilization should be rewarmed to avoid complications associated with low body temperature (including increased mortality, brain injury, hypoglycemia, and respiratory distress). Evidence suggests that warming can be done rapidly (0.5°C/h) or slowly (less than 0.5°C/h) with no significant difference in outcomes.15–19 Caution should be taken to avoid overheating.
Recommendation-Specific Supportive Text
- In a meta-analysis of 8 RCTs involving 1344 term and late preterm infants with moderate-to-severe encephalopathy and evidence of intrapartum asphyxia, therapeutic hypothermia resulted in a significant reduction in the combined outcome of mortality or major neurodevelopmental disability to 18 months of age (odds ratio 0.75; 95% CI, 0.68–0.83).1
- Newly born infants who required advanced resuscitation are at significant risk of developing moderate-to-severe HIE2–4 and other morbidities.5–7
- Newly born infants with abnormal glucose levels (both low and high) are at increased risk for brain injury and adverse outcomes after a hypoxic-ischemic insult.8–14
- Two small RCTs16,19 and 4 observational studies15,17,18,20 of infants with hypothermia after delivery room stabilization found no difference between rapid or slow rewarming for outcomes of mortality,15,17 convulsions/seizures,19 intraventricular or pulmonary hemorrhage,15,17,19,20 hypoglycemia,16,17,19 or apnea.16,17,19 One observational study found less respiratory distress in infants who were slowly rewarmed,18 while a separate study found less respiratory distress syndrome in infants who were rapidly rewarmed.17
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-EO | |
| 1 | C-LD |
|
| 2a | C-EO |
Synopsis
Expert neonatal and bioethical committees have agreed that, in certain clinical conditions, it is reasonable not to initiate or to discontinue life-sustaining efforts while continuing to provide supportive care for babies and families.1,2,4
If the heart rate remains undetectable and all steps of resuscitation have been completed, it may be reasonable to redirect goals of care. Case series show small numbers of intact survivors after 20 minutes of no detectable heart rate. The decision to continue or discontinue resuscitative efforts should be individualized and should be considered at about 20 minutes after birth. Variables to be considered may include whether the resuscitation was considered optimal, availability of advanced neonatal care (such as therapeutic hypothermia), specific circumstances before delivery, and wishes expressed by the family.3,6
Some babies are so sick or immature at birth that survival is unlikely, even if neonatal resuscitation and intensive care are provided. In addition, some conditions are so severe that the burdens of the illness and treatment greatly outweigh the likelihood of survival or a healthy outcome. If it is possible to identify such conditions at or before birth, it is reasonable not to initiate resuscitative efforts. These situations benefit from expert consultation, parental involvement in decision-making, and, if indicated, a palliative care plan.1,2,4–6
Recommendation-Specific Supportive Text
- It is the expert opinion of national medical societies that conditions exist for which it is reasonable to not initiate resuscitation or to discontinue resuscitation once these conditions are identified.1,2,4,5
- Randomized controlled studies and observational studies in settings where therapeutic hypothermia is available (with very low certainty of evidence) describe variable rates of survival without moderate-to-severe disability in babies who achieve ROSC after 10 minutes or more despite continued resuscitation. None of these studies evaluate outcomes of resuscitation that extends beyond 20 minutes of age, by which time the likelihood of intact survival was very low. The studies were too heterogeneous to be amenable to meta-analysis.3
- Conditions in which noninitiation or discontinuation of resuscitation may be considered include extremely preterm birth and certain severe congenital anomalies. National guidelines recommend individualization of parent-informed decisions based on social, maternal, and fetal/neonatal factors.1,2,4 A systematic review showed that international guidelines variably described periviability between 22 and 24 weeks’ gestational age.7
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD |
|
Synopsis
To perform neonatal resuscitation effectively, individual providers and teams need training in the required knowledge, skills, and behaviors. Historically, the repeat training has occurred every 2 years.6–9 However, adult, pediatric, and neonatal studies suggest that without practice, CPR knowledge and skills decay within 3 to 12 months10–12 after training. Short, frequent practice (booster training) has been shown to improve neonatal resuscitation outcomes.5 Educational programs and perinatal facilities should develop strategies to ensure that individual and team training is frequent enough to sustain knowledge and skills.
Recommendation-Specific Supportive Text
- In a randomized controlled simulation study, medical students who underwent booster training retained improved neonatal intubation skills over a 6-week period compared with medical students who did not receive booster training. There was no difference in neonatal intubation performance after weekly booster practice for 4 weeks compared with daily booster practice for 4 consecutive days.1
In a randomized controlled simulation study, pediatric and family practice residents who underwent booster training 9 months after an initial Neonatal Resuscitation Program course demonstrated better procedural skills and teamwork behaviors at a follow-up assessment at 16 months compared with residents who did not receive booster training.2
In a prospective cohort study, physicians and nurses trained in Helping Babies Breathe demonstrated a rapid loss of resuscitation skills by 1 month after training. Subjects who received monthly practice sessions were more likely to pass an objective structured clinical evaluation than those who practiced less frequently.3
In a prospective observational study, implementation of weekly, brief Helping Babies Breathe simulation training after a 1-day Helping Babies Breathe training course resulted in increased frequency of stimulation of newborns, decrease in bag-mask ventilation, and decreased neonatal mortality at 24 hours.4
| COR | LOE | Recommendations |
|---|---|---|
| 2b | C-LD |
|
Synopsis
Briefing has been defined as “a discussion about an event that is yet to happen to prepare those who will be involved and thereby reduce the risk of failure or harm.”4 Debriefing has been defined as “a discussion of actions and thought processes after an event to promote reflective learning and improve clinical performance”5 or “a facilitated discussion of a clinical event focused on learning and performance improvement.”6 Briefing and debriefing have been recommended for neonatal resuscitation training since 20107 and have been shown to improve a variety of educational and clinical outcomes in neonatal, pediatric, and adult simulation-based and clinical studies. The effect of briefing and debriefing on longer-term and critical outcomes remains uncertain.
Recommendation-Specific Supportive Text
Multiple clinical and simulation studies examining briefings or debriefings of resuscitation team performance have shown improved knowledge or skills.8–12
- In a prospective interventional clinical study, video-based debriefing of neonatal resuscitations was associated with improved preparation and adherence to the initial steps of the Neonatal Resuscitation Algorithm, improved quality of PPV, and improved team function and communication.1
In 2 pre–quality improvement/post–quality improvement initiatives, use of a team briefing, debriefing, and predelivery checklist was associated with an improvement in team communication in the delivery room and short-term clinical outcomes, such as decreased frequency of intubation in the delivery room and increased frequency of normothermia on admission to the neonatal intensive care unit. There was no significant effect on other in-hospital clinical outcomes such as bronchopulmonary dysplasia, necrotizing enterocolitis, retinopathy of prematurity, intraventricular hemorrhage, or length of stay.2,3
Neonatal resuscitation science has advanced significantly over the past 3 decades, with contributions by many researchers in laboratories, in the delivery room, and in other clinical settings. While this research has led to substantial improvements in the Neonatal Resuscitation Algorithm, it has also highlighted that we still have more to learn to optimize resuscitation for both preterm and term infants. With growing enthusiasm for clinical studies in neonatology, elements of the Neonatal Resuscitation Algorithm continue to evolve as new evidence emerges.
The current guidelines have focused on clinical activities described in the resuscitation algorithm, rather than on the most appropriate devices for each step. Reviews in 2021 and later will address choice of devices and aids, including those required for ventilation (T-piece, self-inflating bag, flow-inflating bag), ventilation interface (face mask, laryngeal mask), suction (bulb syringe, meconium aspirator), monitoring (respiratory function monitors, heart rate monitoring, near infrared spectroscopy), feedback, and documentation.
Review of the knowledge chunks during this update identified numerous questions and practices for which evidence was weak, uncertain, or absent. The following knowledge gaps require further research:
Resuscitation Preparedness
- The frequency and format of booster training or refresher training that best supports retention of neonatal resuscitation knowledge, technical skills, and behavioral skills
- The effects of briefing and debriefing on team performance
During and Just After Delivery
- Optimal cord management strategies for various populations, including nonvigorous infants and those with congenital heart or lung disease
- Optimal management of nonvigorous infants with MSAF
Early Resuscitation
- The most effective device(s) and interface(s) for providing PPV
- Impact of routine use of the ECG during neonatal resuscitation on resuscitation
- Feasibility and effectiveness of new technologies for rapid heart rate measurement (such as electric, ultrasonic, or optical devices)
- Optimal oxygen management during and after resuscitation
Advanced Resuscitation
- Novel techniques for effective delivery of CPR, such as chest compressions accompanied by sustained inflation
- Optimal timing, dosing, dose interval, and delivery routes for epinephrine or other vasoactive drugs, including earlier use in very depressed newly born infants
- Indications for volume expansion, as well as optimal dosing, timing, and type of volume
- The management of pulseless electric activity
Specific Populations
- Management of the preterm newborn during and after resuscitation
- Management of congenital anomalies of the heart and lungs during and after resuscitation
- Resuscitation of newborns in the neonatal unit after the newly born period
- Resuscitation of newborns in other settings up to 28 days of age
Postresuscitation Care
- Optimal dose, route, and timing of surfactant in at-risk newborns, including less-invasive administration techniques
- Indications for therapeutic hypothermia in babies with mild HIE and in those born at less than 36 weeks' gestational age
- Adjunctive therapies to therapeutic hypothermia
- Optimal management of blood glucose
- Optimal rewarming strategy for newly born infants with unintentional hypothermia
For all these gaps, it is important that we have information on outcomes considered critical or important by both healthcare providers and families of newborn infants.
The research community needs to address the paucity of educational studies that provide outcomes with a high level of certainty. Internal validity might be better addressed by clearly defined primary outcomes, appropriate sample sizes, relevant and timed interventions and controls, and time series analyses in implementation studies. External validity might be improved by studying the relevant learner or provider populations and by measuring the impact on critical patient and system outcomes rather than limiting study to learner outcomes.
Researchers studying these gaps may need to consider innovations in clinical trial design; examples include pragmatic study designs and novel consent processes. As mortality and severe morbidities decline with biomedical advancements and improvements in healthcare delivery, there is decreased ability to have adequate power for some clinical questions using traditional individual patient randomized trials. Another barrier is the difficulty in obtaining antenatal consent for clinical trials in the delivery room. Adaptive trials, comparative effectiveness designs, and those using cluster randomization may be suitable for some questions, such as the best approach for MSAF in nonvigorous infants. High-quality observational studies of large populations may also add to the evidence. When feasible, well-designed multicenter randomized clinical trials are still optimal to generate the highest-quality evidence.
Finally, we wish to reinforce the importance of addressing the values and preferences of our key stakeholders, the families and teams who are involved in the process of resuscitation. Gaps in this domain, whether perceived or real, should be addressed at every stage in our research, educational, and clinical activities.
The American Heart Association requests that this document be cited as follows: Aziz K, Lee HC, Escobedo MB, Hoover AV, Kamath-Rayne BD, Kapadia VS, Magid DJ, Niermeyer S, Schmolzer GM, Szyld E, Weiner GM, Wyckoff MH, Yamada NK, Zaichkin J. Part 5: neonatal resuscitation: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142(suppl 2):S524–S550. doi: 10.1161/ CIR.0000000000000902
This article has been copublished in Pediatrics.
- Khalid Aziz, MBBS, MA, MEd(IT), Chair
- Henry C. Lee, MD, Vice Chair
- Marilyn B. Escobedo, MD
- Amber V. Hoover, RN, MSN
- Beena D. Kamath-Rayne, MD, MPH
- Vishal S. Kapadia, MD, MSCS
- David J. Magid, MD, MPH
- Susan Niermeyer, MD, MPH
- Georg M. Schmölzer, MD, PhD
- Edgardo Szyld, MD, MSc
- Gary M. Weiner, MD
- Myra H. Wyckoff, MD
- Nicole K. Yamada, MD, MS
- Jeanette Zaichkin, RN, MN, NNP-BC
We thank Dr. Abhrajit Ganguly for assistance in manuscript preparation.
Open table in a new window.
Open table in a new window.
 Login
Login